Urodynamics do not always give clear information and have limitations, not least for those who cannot urinate at all or who use a catheter. The waiting time for examination is often long.
CoreFlow® can replace / supplement a urodynamic examination and also be used when urodynamics alone are not applicable, for example if the patient can not urinate or use a catheter.
Many men with benign prostate enlargement use CAD (indwelling catheter) to be able to empty the bladder.
Not being able to empty the bladder and/or be treated with CAD entails a risk of urinary tract infections, which in turn lead to both a lot of suffering and great costs.
Reduces the risk of urinary tract infection
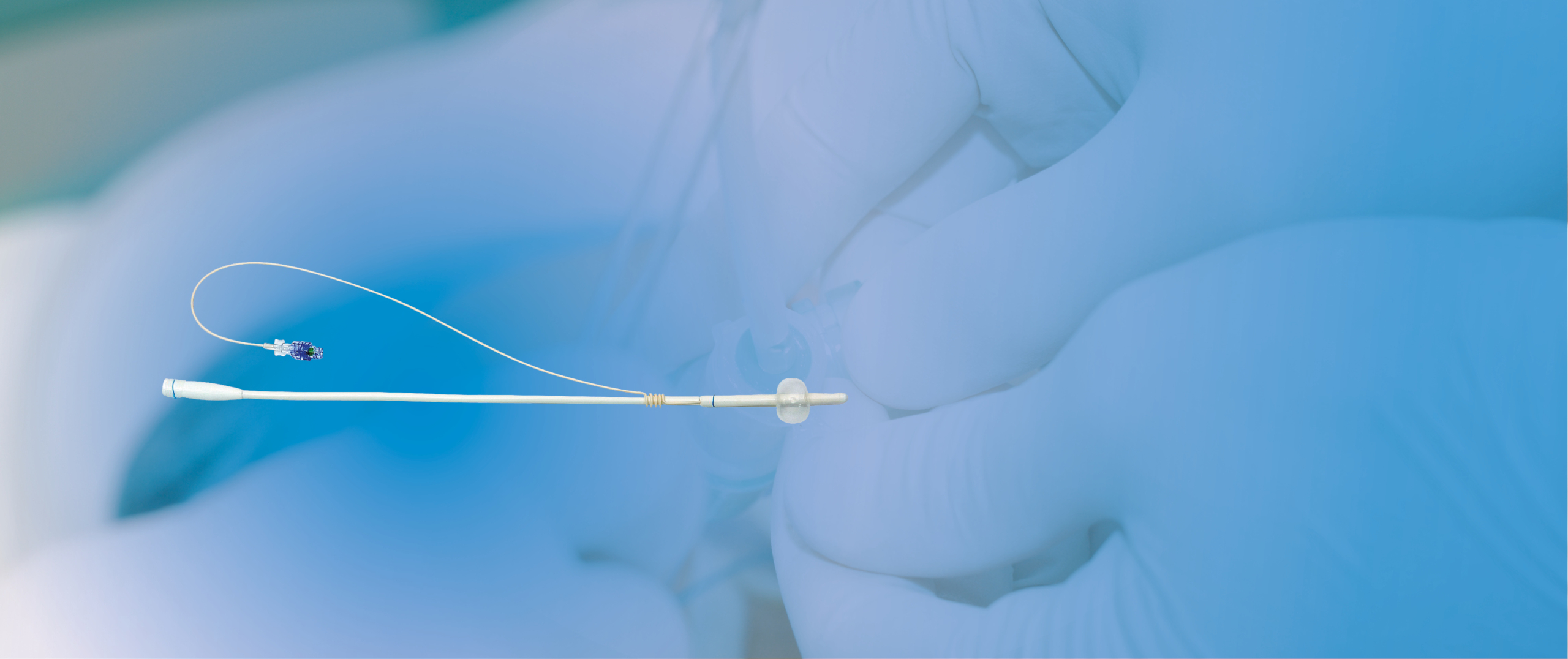
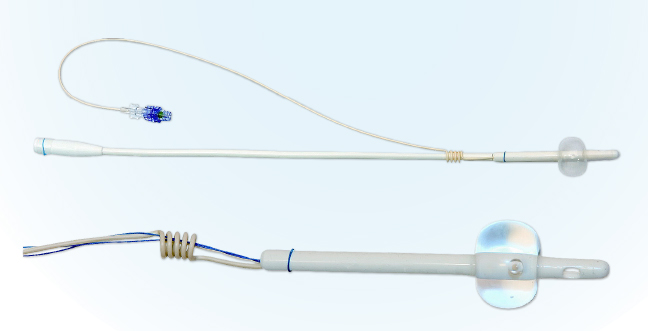
CoreFlow® can replace CAD for men, thereby radically reducing the risk of urinary tract infection. When the rear section of CoreFlow® is separated, the patient urinates on his own or use self-catheterisation by pulling the thread. The risk of bacterial growth and invasion via the urethra will be minimal. In addition, patients do not have to use a urine bag/valve linked to a CAD but urinate on their own, which has a major impact on quality of life. For patients who have undergone minimally invasive treatments, such as thermotherapy treatment with CoreTherm®, CoreFlow® is suitable to use as relief until the swelling has decreased. Patients can urinate on their own right from day zero. The risk of catheter-associated infections is thereby reduced.
Diagnostic function
CoreFlow® used as a temporary prostatic stent simulates how the urination/treatment result will be after thermotherapy/surgery for a BPE patient. CoreFlow® can thus replace/supplement a urodynamic examination and be used when urodynamics alone is not applicable, for example if the patient cannot urinate or use a catheter. In addition, CoreFlow® can confirm that the patient is continent with only the outer sphincter if the inner sphincter is kept open while temporarily eliminating the obstruction.
Free up resources in healthcare
Urodynamic examinations are time-consuming and cost-driven, not infrequently the waiting times are also long. CoreFlow® can replace the urodynamic examination and be used where the latter does not work. CoreFlow® can save resources for healthcare and shorten the time to cure treatment for the patient with benign prostate enlargement.